Clean, renewable and plentiful, hydrogen can be harnessed to fuel the modern world. The question scientists have come up against in the past is how to store suitable quantities of hydrogen safely while still allowing for its release in a timely and economically viable manner. The storage of hydrogen in solid form, such as MgH2 (magnesium hydride) is a technique that is being developed for deployment in hydrogen stations for industrial and vehicular applications with minimal associated pollution.
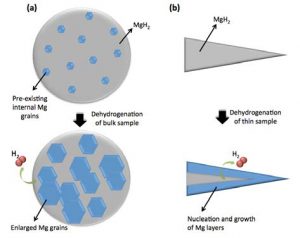
MgH2 is a combination of magnesium and hydrogen. The mechanisms by which hydrogen is added (hydriding) and removed (dehydriding) from the magnesium has long been the subject of debate. The common belief in the past has been that dehydriding hydrogen from magnesium occurred in line with a ‘shrinking core’ model, in which hydrogen atoms are released from the surface of the hydride particles. This belief has been based on results gleaned from studying extremely thin samples or nanoparticles. Researchers at The University of Queensland, with the support of Professional Officers Dr Wael Al Abdulla, Dr Javaid Khan and Mr Kai-Yu Liu at ANFF-Q, have recently provided new evidence that this common belief is not true for bulk materials, which are used in industrial scale hydrogen storage systems. Their project, led by Associate Professor Kazuhiro Nogita, has shown that in bulk MgH2, dehydriding takes place by a process of nucleation and growth of magnesium grains, inside the bulk hydride particles but not from the surface of the particles. This finding will influence significantly the system operation conditions of hydrogen storage systems.
Using advanced technology including the differential scanning calorimeter at ANFF-Q, the ultra-high voltage transmission electron microscope at Kyushu University in Japan and the synchrotron powder x-ray diffraction at the Australian Synchrotron, the research team were able to observe the behaviour of hydrogen release from bulk materials in real time.
This unprecedented discovery led to the publication of “Evidence of the hydrogen release mechanism in bulk MgH2” in Scientific Reports*, a high-impact journal published by the Nature publication group. This work will contribute significantly towards the development of large scale commercial hydrogen storage systems, and therefore towards efficient and safe hydrogen filling stations for fuel cell vehicles in modern transport systems.





